ADVERTISEMENT
Cardiac Catheterization in Patients With Ascending Aortic Aneurysms: Safety, Success, and Prevalence of Coronary Artery Disease
Abstract: Background. Evaluation for coronary artery disease (CAD) is recommended prior to surgery for ascending aortic aneurysms. Concerns regarding the use of coronary angiography in this population include safety and the ability to successfully selectively engage the coronary arteries. Additionally, the prevalence of CAD is not well described. Methods. We retrospectively reviewed all patients referred for cardiac catheterization prior to elective surgery for an ascending aortic aneurysm at our institution over a 4-year period. Catheter selection was based on knowledge of the aneurysm size. Images were screened for whether selective coronary engagement was achieved and for the presence of significant coronary disease. Results. A total of 205 patients met the inclusion criteria. The mean age was 61 years and 63% were male. There were no adverse events related to catheterization. The left coronary artery was selectively engaged in 98% of patients, and the right coronary in 92%. On average, 3.1 catheters were used for angiography per patient. Coronary artery disease was present in 19% of patients (n = 39). Increasing age was the only risk factor significantly associated with the presence of disease. Coronary bypass was required in 15% of patients at the time of aortic aneurysm surgery. Conclusions. Coronary angiography can be performed safely and the coronary arteries can be successfully selectively engaged in patients with ascending aortic aneurysms. The findings frequently impact the surgical approach. We believe that coronary angiography should be part of the routine preoperative evaluation in appropriate patients.
J INVASIVE CARDIOL 2014;26(6):241-244
Key words: coronary angiography, ascending aortic aneurysm
_____________________________________
Surgery to repair ascending aortic aneurysms is increasingly common, and is intended to reduce the risk of rupture and the associated high mortality rate of such events.1 Pre-operative evaluation for coronary artery disease (CAD), such as with cardiac catheterization, is recommended before such surgical procedures, much as it is prior to other elective open-heart repair, such as surgical treatment of valvular heart disease.1,2 If significant coronary disease is identified, it can be treated with coronary artery bypass at the time of surgery, often utilizing the same surgical approach.
There are several important differences when cardiac catheterization is performed in patients with ascending aortic aneurysms compared to other patients. Catheterization in the presence of an ascending aortic aneurysm may entail a higher risk of dissection due to the fragile aortic wall.3 The coronary arteries may be more difficult to selectively engage, given the distorted anatomy of the aortic root where the catheters must be manipulated, and catheters that are less commonly used may be required.4 Lastly, the incidence of coronary disease in patients with ascending aortic aneurysm is not well-defined. If it is lower than for other patients being prepared for cardiac surgery, it would reduce the likelihood of the catheterization impacting the ultimate plan of care.5
We undertook the present study to answer three basic questions about cardiac catheterization and coronary angiography in patients with ascending aortic aneurysms and a plan for surgical repair: (1) Is cardiac catheterization safe? (2) Can cardiac catheterization be performed effectively (ie, can the coronary arteries be selectively engaged on a reliable basis)? and (3) What is the incidence of significant CAD in this population?
Methods
We performed a retrospective cohort analysis of all patients with ascending aortic aneurysm and a plan for surgical repair who presented to the cardiac catheterization laboratory at the Hospital of the University of Pennsylvania between January 1, 2007 and December 31, 2010. The study was approved by the University’s Institutional Review Board.
Patient selection. All patients with ascending aortic aneurysm and a plan for elective surgical repair who presented to our cardiac catheterization laboratory were screened for inclusion. Patients ages 18 years and older were included in the review. Patients undergoing emergent cardiac catheterization and patients with aortic dissection were excluded. At our institution, preoperative cardiac catheterization is generally performed in males if they are 30 years of age or older and females 40 years of age or older. Younger patients are referred for catheterization if they are considered to be at increased risk for coronary disease. Final decisions as to the need for cardiac catheterization were made by the referring surgeon. Final decisions regarding the need for bypass surgery were made by the invasive cardiologist and referring surgeon in consultation.
Cardiac catheterization technique. Left heart catheterization and coronary angiography were performed using standard techniques. Procedures were performed through the femoral artery using 6 Fr systems, unless contraindicated. For the left coronary artery, we primarily used Judkins catheters. Catheter size was determined by the diameter of the aortic aneurysm as follows. For aneurysms less than 5.5 cm, a Judkins Left (JL) 4 or JL5 was used as the first choice. For aneurysms larger than 5.5 cm, a JL6 or JL7 was the most common initial catheter. For the right coronary artery, a Judkins Right (JR) 4 was the initial catheter choice. Depending on the location of the right coronary and whether the take-off was superiorly or inferiorly directed, the second choice catheter was typically an Amplatz type catheter or a Castillo type catheter. Aortography was performed at the discretion of the invasive cardiologist.
Statistical analysis. The primary adverse safety event was aortic dissection. Other adverse events were any catheter-induced complication requiring urgent surgical repair, or angiographic evidence of coronary dissection, regardless of whether coronary intervention was required. All catheterization films were reviewed by a research cardiologist. Selective engagement of the left coronary artery and right coronary artery were noted. We defined complete success as selective engagement of both coronary arteries, and failure as the inability to selectively engage either coronary artery. We prospectively defined 5.5 cm as a cutpoint to compare catheterization techniques in larger vs smaller aneurysms. Coronary artery disease was defined as a stenosis >70% in any epicardial coronary artery >2 mm in diameter, or a stenosis >50% in the left main coronary artery. Comparisons between patient populations were made using Fisher’s exact test for categorical variables, and a student’s t-test for continuous variables with parametric distributions. Non-parametric variables were compared with a Mann-Whitney U-test. All samples were independent. To determine independent risk factors for coronary disease, all variables with a univariate P-valve <.10 were included in a binary logistic regression model. P-values <.05 were considered significant, and all tests of significance were two-sided.
Results
A total of 205 patients with ascending aortic aneurysm and a plan for surgery were referred for catheterization. Baseline patient characteristics are summarized in Table 1. The mean age was 61 years, 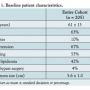 and 63% were male. The mean aneurysm size was 5.6 cm. Nine patients had prior coronary bypass surgery.
and 63% were male. The mean aneurysm size was 5.6 cm. Nine patients had prior coronary bypass surgery.
Safety and success of cardiac catheterization. Cardiac catheterization was performed via the femoral approach in 201 patients, the radial approach in 3 patients, and the brachial approach in 1 patient. There were no adverse events associated with cardiac catheterization. No patient experienced a dissection, either of the aorta or of a coronary artery. All patients were able to proceed with surgery as planned.
Details of the cardiac catheterization are shown in Table 2. Overall, both coronary arteries were able to be selectively engaged in 90% of patients (n = 185). There were no patient characteristics that predicted successful coronary engagement. Specifically, age, aneurysm size, or gender did not predict the ability to cannulate the coronary arteries. The average total number of catheters used per patient was 3.1. Fluoroscopy time (20 ± 10 min vs 13 ± 7 min; P<.001) and total number of catheters used (4.7 ± 1.8 vs 2.9 ± 1.2; P<.001) were higher for patients in whom there was a failure to 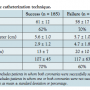 selectively engage either coronary artery. Average contrast use was 118 mL when aortography was performed, and 85 mL when aortography was not performed.
selectively engage either coronary artery. Average contrast use was 118 mL when aortography was performed, and 85 mL when aortography was not performed.
The left coronary artery was successfully selectively engaged in 98% of patients (n = 201). An average of 1.88 catheters were used per patient for the left coronary artery. Catheter use is shown in Table 3. The most commonly used catheters were JL4, JL5, and JL6. Compared to patients with smaller aneurysms (≤5.5 cm), there was a trend toward higher catheter use for the left coronary in patients with larger aneurysms (1.91 vs 1.65; P=.07). The right coronary artery was successfully selectively engaged in 92% of attempted patients (n = 183 out of 200). In 5 patients with known occluded right coronary arteries, no attempt was made. An average of 1.3 catheters were used per patient for engagement of the right coronary artery. The JR4 catheter was attempted in 189 patients and was successful in 78% (n = 147) (Table 3). There was no difference in terms of the number of catheters used for the right coronary artery for patients with large vs small ascending aortic aneurysms (1.33 vs 1.30; P=.76).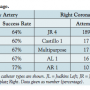
Presence of coronary artery disease. Coronary artery disease was present in 19% of patients (n = 39). Of these, 77% underwent coronary artery bypass at the time of aortic aneurysm surgery (n = 30), representing 15% of the entire cohort. As seen in Table 4, age was the only risk factor significantly associated with CAD, although each of the traditional CAD risk factors was more prevalent in the group of patients with disease. When age and hypertension were included in a binary logistic regression analysis, age remained a significant predictor of coronary disease (odds ratio, 1.33 per 5-year age increase; 95% confidence interval, 1.13-1.56; P=.01). Prior bypass surgery was excluded from the regression as these were patients with known coronary disease. When the need to perform bypass surgery during aortic aneurysm surgery was used as the dependent variable instead of the presence of CAD, the results were unchanged.
Discussion
We performed this study to address three concerns with coronary angiography in patients with ascending aortic aneurysm, namely the safety of the procedure, the success of coronary engagement, and the presence of coronary disease. To our knowledge, this is the largest study of cardiac catheterization in patients with ascending aortic aneurysm. We showed that coronary angiography can be performed safely and successfully in this population, and the incidence of CAD was 19%.
Safety of cardiac catheterization. In over 200 procedures, we had no adverse events related to the performance of coronary angiography. Specifically, there were no aortic aneurysm, sinus, or ostial coronary dissections. Our findings are similar to those of Israel et al, who had no such complications in their study of 63 patients.4 In Ueda et al’s study of 59 patients with thoracic aortic aneurysms undergoing catheterization, 3 patients (5%) had adverse events related to cardiac catheterization that rendered them no longer surgical candidates, but the exact nature of those events is not described.3
While dissections are exceedingly rare during the average diagnostic catheterization,6 there is cause for heightened safety concerns in patients with aneurysms of the ascending aorta. When catheterization is performed in a patient with a dissecting aortic aneurysm, there is a risk of propagating that dissection.7 Even in patients without aortic dissection, increased catheter manipulation and variant coronary ostia distorted by the aneurysm may lead to increased risk of coronary dissection.8 Still, our study shows that when performed carefully, coronary angiography in these patients is safe.
Successful selective coronary engagement. Successful selective engagement of the coronary arteries is clearly more difficult in patients with ascending aortic aneurysm, although our success rates overall were high. We were unable to selectively engage the left coronary artery only 2% of the time, and the right coronary artery only 8% of the time. Our results are similar to those of Israel et al,4 the only other study to report on the technical aspects of cardiac catheterization in this population. In that study, selective engagement was not achieved in 3 out of 63 patients with ascending aortic aneurysm (1 left coronary, 2 right coronary), but clearly higher than the failure rate of <1% in studies of patients with normal anatomy.9,10 We required 3.1 catheters per patient for angiography from the femoral approach, which is higher than in patients with normal anatomy, who only require an average of about 2.1.9,10 Fluoroscopy time and contrast volume were also higher than would be expected in patients with normal anatomy.
Perhaps the most important technical contributions of our study were showing that selective coronary angiography can be successfully performed using standard catheter shapes and sizes and can be accomplished using 6 Fr arterial sheaths. Judkins catheters are the standard first choice catheters for coronary angiography from the femoral approach, and using these we were successful the vast majority of the time. This is in contradistinction to the study of Israel et al, which frequently made use of a Nesto catheter (which may not be available currently in most catheterization laboratories), and 8 Fr sheaths.4 Reducing vascular access sheath size has been shown to reduce access-site complications.11 In addition, our catheter size selection algorithm was based on knowledge of the preoperative computed tomography scan, thus eliminating the need for aortography as was done in the study by Israel. Although there has been increasing use of radial artery catheterization for coronary angiography,12 we primarily chose femoral access in our study for several reasons. A right radial approach would likely lower the success rate for engaging the left coronary artery, as that radial approach biases toward the lesser curvature of the aorta. The left radial approach would likely allow for similar catheter selection as in our study, although this approach is less convenient for the operator. Either radial approach may be more difficult if the thoracic aneurysm distorts the entry of the subclavian artery into the aorta.
Presence of coronary artery disease. Testing for coronary disease is recommended prior to thoracic aortic surgery.1 Whether this should occur routinely for all patients or only for symptomatic individuals and for those with positive non-invasive testing remains a matter of debate.13,14 Accurate detection of coronary disease is important. For example in a study by Ueda et al, unrevascularized significant coronary artery disease was the only predictor of perioperative myocardial infarction, a frequently fatal complication, in patients undergoing thoracic aneurysm surgery.3 Many programs, including our own, routinely perform pre-operative coronary angiography unless contraindicated.
We found coronary disease in 19% of our patients, and bypass was required in 15%. Similarly, Kieffer et al. found significant coronary disease in 26% of asymptomatic patients with thoracic aortic aneurysms.5 Martin et al. evaluated 133 patients undergoing elective repair of thoracoabdominal aortic aneurysms and found that 26% required coronary revascularization prior to surgery.15 The 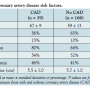 prevalence of coronary disease in the thoracic aortic aneurysm population is similar to that in patients with valvular heart disease, where a recent studied suggested a prevalence of 26% in patients being prepared for surgery.16 Indeed, coronary angiography is routinely indicated for patients undergoing surgery for valvular heart disease.2 This is supportive of the argument that cardiac catheterization is warranted prior to ascending aortic aneurysm surgery just as it is prior to valvular surgery.
prevalence of coronary disease in the thoracic aortic aneurysm population is similar to that in patients with valvular heart disease, where a recent studied suggested a prevalence of 26% in patients being prepared for surgery.16 Indeed, coronary angiography is routinely indicated for patients undergoing surgery for valvular heart disease.2 This is supportive of the argument that cardiac catheterization is warranted prior to ascending aortic aneurysm surgery just as it is prior to valvular surgery.
There is another option for assessing coronary disease that may be particularly suited to patients with ascending aortic aneurysms. Frequently, patients with aneurysms undergo CT scanning to determine the aneurysm size and other characteristics. Incorporating CT angiography of the coronary arteries into a planned CT scan of the aorta could potentially combine both evaluations into one test, sparing patients the additional risks of cardiac catheterization.17 Whether such a strategy could perform as well as routine cardiac catheterization will require further study.
Study limitations. This study is limited by its size and by being a single-institution experience. Adverse events that may occur a small percentage of the time may not have occurred in our study due to chance. Although no neurologic complications were noted on routine post-catheterization examination, detailed neurologic examination or imaging was not routinely performed and small embolic events may not have been detected. Furthermore, while the prevalence of coronary disease in this study fits with existing data, determining the importance of traditional risk factors is difficult with our sample size. Nevertheless, to our knowledge, this is the largest study of cardiac catheterization in patients with ascending aortic aneurysms.
Conclusion
We conducted a retrospective analysis of cardiac catheterization in patients with ascending aortic aneurysm and a plan for surgical repair. We found that cardiac catheterization and coronary angiography can be performed safely, and that selective engagement of the coronary arteries was achieved in 98% of left coronaries and 92% of right coronary arteries. Knowledge of the aneurysm size based on preoperative computed tomography scan was useful in selecting catheter size. Significant coronary disease was found in 19% of patients, and 15% of patients required bypass surgery at the time of aneurysm repair. We believe that cardiac catheterization and coronary angiography should be part of the routine preoperative evaluation in appropriate patients.
References
- Hiratzka LF, Bakris GL, Beckman JA, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with thoracic aortic disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. Circulation. 2010;121(13):e266-e369.
- Bonow RO, Carabello BA, Chatterjee K, et al. 2008 focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to develop guidelines for the management of patients with valvular heart disease). Circulation. 2008;118(15):e523-e661.
- Ueda T, Shimizu H, Shin H, et al. Detection and management of concomitant coronary artery disease in patients undergoing thoracic aortic surgery. Jpn J Thorac Cardiovasc Surg. 2001;49(7):424-430.
- Israel DH, Sharma SK, Ambrose JA, Ergin MA, Griepp RR. Cardiac catheterization and selective coronary angiography in ascending aortic aneurysm or dissection. Cathet Cardiovasc Diagn. 1994;32(3):232-237.
- Kieffer E, Chiche L, Baron JF, Godet G, Koskas F, Bahnini A. Coronary and carotid artery disease in patients with degenerative aneurysm of the descending thoracic or thoracoabdominal aorta: prevalence and impact on operative mortality. Ann Vasc Surg. 2002;16(6):679-684.
- Perez-Castellano N, Miguel A. Garcia-Fernandez MA, et al. Dissection of the aortic sinus of Valsalva complicating coronary catheterization: cause, mechanism, evolution, and management. Cathet Cardiovasc Diagn. 1998;43(3):273-279.
- Hart WL, Berman EJ, Lacom RJ. Hazard of retrograde aortography in dissecting aneurysm. Circulation. 1963;27:1140-1142.
- Boyle AJ, Chan M, Dib J, Resar J. Catheter-induced coronary artery dissection: risk factors, prevention and management. J Invasive Cardiol. 2006;18(10):500-503.
- Cooper CJ, El-Shiekh RA, Cohen DJ, et al. Effect of transradial access on quality of life and cost of cardiac catheterization: a randomized comparison. Am Heart J. 1999;138(3 Pt 1):430-436.
- Louvard Y, Lefévre T, Allain A, Morice MC. Coronary angiography through the radial or the femoral approach: the CARAFE study. Catheter Cardiovasc Interv. 2001;52(2):181-187.
- Muller DWM, Shamir KJ, Ellis SG, Topol EJ. Peripheral vascular complications after conventional and complex percutaneous coronary interventional procedures. Am J Cardiol 1992;69(1):63-68.
- Abbott JD. Diffusion of innovations and adoption of transradial intervention. Circ Cardiovasc Interv. 2013;6(3):199-200.
- Kouchoukos NT, Dougenis D. Surgery of the thoracic aorta. N Engl J Med. 1997;336(26):1876-1888.
- Greenberg R, Risher W. Clinical decision making and operative approaches to thoracic aortic aneurysms. Surg Clin North Am. 1998;78(5):805-826.
- Martin GH, O’Hara PJ, Hertzer NR, et al. Surgical repair of aneurysms involving the suprarenal, visceral, and lower thoracic aortic segments: early results and late outcome. J Vasc Surg. 2000;31(5):851-862.
- Meijboom WB, Mollet NR, Van Mieghem CAG, et al. Pre-operative computed tomography coronary angiography to detect significant coronary artery disease in patients referred for cardiac valve surgery. J Am Coll Cardiol. 2006;48(8):1658-1665.
- Johnson TRC, Nikolaou K, Becker A, et al. Dual-source CT for chest pain assessment. Eur Radiol. 2008;18(4):773-780.
_______________________________
From the 1Division of Cardiology, Virginia Commonwealth University Medical Center, Richmond, Virginia; 2Division of Cardiology, Hospital of the University of Pennsylvania, Philadelphia, Pennsylvania; and 3Division of Cardiovascular Surgery, Hospital of the University of Pennsylvania, Philadelphia, Pennsylvania.
Disclosure: The authors have completed and returned the ICMJE Form for Disclosure of Potential Conflicts of Interest. The authors report no conflicts of interest regarding the content herein.
Manuscript submitted September 30, 2013, provisional acceptance given December 4, 2013, final version accepted December 9, 2013.
Address for correspondence: Zachary M. Gertz, MD, 1200 East Broad St, West Hospital, 5th Floor, West Wing, Room 529-B, Richmond, VA 23298. Email: zgertz@mcvh-vcu.edu



















