ADVERTISEMENT
Gender-Based Analysis of the 3-Year Outcome of Bioactive Stents Versus Paclitaxel-Eluting Stents in Patients with Acute Myocardial Infarction: An Insight from the TITAX-AMI Trial
Abstract: Background. The TITAX-AMI trial demonstrated a better clinical outcome of titanium-nitride-oxide-coated bioactive stents (BAS) as compared with paclitaxel-eluting stents (PES) in patients with acute myocardial infarction (MI) undergoing early percutaneous coronary intervention (PCI). We explored the gender-based 3-year outcome of BAS as compared with PES in a subgroup analysis of the TITAX-AMI trial. Methods. A total of 214 patients (52 women) with acute MI were randomly assigned to BAS, and 211 patients (54 women) to PES. The primary endpoint was major adverse cardiac events (MACE) including cardiac death, recurrent MI, and target lesion revascularization (TLR). Secondary endpoints were all-cause death, a composite of cardiac death or recurrent MI, and stent thrombosis (ST). Results. Women were older and had smaller reference vessel diameter (P<.001 for both) as compared with men. At 3-year follow-up, both MACE and TLR showed a trend to be higher in women as compared with men (24.5% versus 16.3% [P=.059] and 15.1% versus 8.8% [P=.065], respectively). The rate of all-cause death was significantly higher in women as compared with men (13.2% versus 6.0%, respectively; P=.02). Among female patients, MACE, cardiac death, recurrent MI, TLR, and ST were all statistically similar between the two stent groups (P>.05 for all). Conclusions. In the current post hoc gender-based analysis of the TITAX-AMI trial, the 3-year outcome of patients undergoing PCI for acute MI was slightly worse in female patients as compared with their male counterparts, as reflected by a trend toward a higher primary composite endpoint of MACE and TLR.
J INVASIVE CARDIOL 2012;24:104–108
Key words: percutaneous coronary intervention, drug-eluting stents, acute myocardial infarction
___________________________________________
Interventional cardiology has come a long way since the introduction of coronary stents in the 1990s provided a safer approach for percutaneous coronary interventions (PCI).1,2 Overall, women undergoing PCI have more comorbidities, and more often have multivessel disease in predominantly smaller coronaries.3,4 There is some uncertainty, however, as to whether — and how — gender would influence the outcome of coronary stent implantation.
Indeed, the arrival of drug-eluting stents (DES) on the scene has dramatically reshaped the landscape of coronary intervention, resulting in a marked reduction of restenosis rates by one-half to two-thirds at 5-year follow-up.5,6 In patients with stable coronary artery disease, long-term benefit was observed following DES implantation in men and women, alike.4,7 In fact, most randomized trials comparing DES with bare-metal stents (BMS) in the setting of primary PCI for ST-elevation myocardial infarction (MI) showed a reduction of target lesion revascularization (TLR) with DES, without an increase in the incidence of stent thrombosis (ST).8-12 Yet, after years ‘in duty,’ worrisome reports have raised concern about a small, but definite, increase of late and very late ST with the use of DES.13
A further step forward was taken with the design of bioactive stents (BAS). The safety of titanium-nitride-oxide-coated BAS has been established in several reports from real-life unselected populations.14,15 Surprisingly, in the highly challenging realm of acute MI, BAS did achieve a better outcome as compared with the Food and Drug Administration-approved paclitaxel-eluting stent (PES) at 2-year follow-up.16 A lingering question remains as to whether these results will remain consistent in female patients. So far, we explored the gender-based 3-year outcome of BAS as compared with PES in patients presenting with acute MI as evaluated in a subgroup analysis of the Titanium-Nitride-Oxide-Coated Stents versus Paclitaxel-Eluting Stents in Acute Myocardial Infarction (TITAX-AMI) trial.
Methods
Patient selection and study design. The design of the original trial has been previously reported.16,17 Briefly, the TITAX-AMI trial is a prospective randomized multicenter trial in which 425 patients presenting with acute MI were randomized in a 1:1 fashion to receive either Titan-2 BAS (Hexacath) or TAXUS Liberte PES (Boston Scientific). Predilatation of culprit lesion, PCI technique, selection of access site, administration of intravenous heparin, low-molecular-weight heparin, bivalirudin, and glycoprotein IIb/IIIa receptor inhibitors were left to the discretion of the operator. In patients not maintained on aspirin, the study protocol recommended premedication with aspirin at a loading dose of 100-500 mg orally, or 250-500 mg intravenously. Clopidogrel was administered at a loading dose of 300-600 mg orally immediately after the index procedure, if the patient was not already maintained on clopidogrel. At discharge, aspirin was prescribed at a dose of 100 mg daily orally, indefinitely, and clopidogrel at a dose of 75 mg daily orally, for at least 6 months. Clinical follow-up was planned at 1, 6, 12, 24, and 36 months.
Study endpoints and definitions. Diagnostic criteria for non-ST elevation MI and ST-segment elevation MI have been described in detail previously.16,17 The primary endpoint was the first occurrence of major adverse cardiac events (MACE), defined as a composite of cardiac death, recurrent MI, or TLR. Secondary endpoints included all-cause death, a composite of cardiac death or recurrent MI, and ST. The 3-year analysis was prespecified per protocol (follow-up data were planned to be collected yearly for 5 years).
Ethical issues. The study was initiated by the investigators and conducted according to the ethical guidelines of the American Physiological Society. Informed written consent was obtained from every patient after full explanation of the study protocol. The study protocol was approved by the Ethics Committees of the coordinating center, Satakunta Central Hospital, and the participating hospitals. The study has been registered at www.clinicaltrials.gov, under the number NCT00495664.
Statistical analysis. Continuous variables were presented as means ± standard deviations, while categorical variables were described with absolute and relative (percentage) frequencies. Comparisons between the two groups were performed using the unpaired two-tailed t-test for continuous, and the Pearson Chi-square test or Fisher’s exact test for categorical variables. Time-to-event curves were constructed using the Kaplan-Meier method and data were compared using the log-rank test. Univariate and multivariable logistic regression analyses were performed to identify the independent predictors of MACE at 3-year follow-up. All tests were two-sided and statistical significance was set at 5%. All data were analyzed with SPSS version 17.0 (SPSS).
Results
 Baseline, angiographic and procedural characteristics. Of the 425 randomized patients, 214 were assigned to Titan-2 BAS (including 52 women [24.3%]), and 211 to TAXUS Liberte PES (including 54 women [25.6%]). Baseline clinical characteristics were balanced between female and male patients (Table 1). However, women were significantly older and less commonly smokers as compared with men. Diabetes mellitus was present in one-fifth of patients. Baseline angiographic characteristics were also balanced between genders except for a significantly smaller reference vessel diameter in women (Table 2).
Baseline, angiographic and procedural characteristics. Of the 425 randomized patients, 214 were assigned to Titan-2 BAS (including 52 women [24.3%]), and 211 to TAXUS Liberte PES (including 54 women [25.6%]). Baseline clinical characteristics were balanced between female and male patients (Table 1). However, women were significantly older and less commonly smokers as compared with men. Diabetes mellitus was present in one-fifth of patients. Baseline angiographic characteristics were also balanced between genders except for a significantly smaller reference vessel diameter in women (Table 2).
 Clinical outcome of the TITAX-AMI at 3-year follow-up. The 3-year cumulative incidence of MACE was significantly lower in patients assigned to BAS as compared with those assigned to PES (13.1% versus 23.7%, respectively; P=.006). Similarly, the 3-year rates of cardiac death and recurrent MI were significantly lower in patients assigned to BAS (1.4% versus 5.2% and 6.1% versus 16.6%, P=.028 and P=.001, respectively). Nevertheless, the rates of TLR were similar between the two study groups (9.8% versus10.9%, respectively; P=.75). The rate of ST was again significantly lower in patients assigned to BAS (0.5% versus 6.6%, respectively; P<.001).
Clinical outcome of the TITAX-AMI at 3-year follow-up. The 3-year cumulative incidence of MACE was significantly lower in patients assigned to BAS as compared with those assigned to PES (13.1% versus 23.7%, respectively; P=.006). Similarly, the 3-year rates of cardiac death and recurrent MI were significantly lower in patients assigned to BAS (1.4% versus 5.2% and 6.1% versus 16.6%, P=.028 and P=.001, respectively). Nevertheless, the rates of TLR were similar between the two study groups (9.8% versus10.9%, respectively; P=.75). The rate of ST was again significantly lower in patients assigned to BAS (0.5% versus 6.6%, respectively; P<.001).
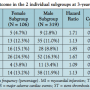 Gender-based 3-year clinical outcome. The 3-year cumulative incidence of MACE trended higher in women as compared with men (24.5% versus 16.3%, respectively; P=.059) (Table 3 and Figure 1). Likewise, TLR rates trended higher in women (15.1% versus 8.8%, respectively; P=.065). Otherwise, the 3-year rates of cardiac death, recurrent MI, and ST were statistically matched between genders. However, the rate of all-cause death was significantly higher in women as compared with men (13.2% versus 6.0%, respectively; P=.02) (Table 3).
Gender-based 3-year clinical outcome. The 3-year cumulative incidence of MACE trended higher in women as compared with men (24.5% versus 16.3%, respectively; P=.059) (Table 3 and Figure 1). Likewise, TLR rates trended higher in women (15.1% versus 8.8%, respectively; P=.065). Otherwise, the 3-year rates of cardiac death, recurrent MI, and ST were statistically matched between genders. However, the rate of all-cause death was significantly higher in women as compared with men (13.2% versus 6.0%, respectively; P=.02) (Table 3).
 Stent-based analysis of the two gender subgroups. Among male patients, the primary composite endpoint of MACE was significantly lower in those assigned to receive BAS as compared with those assigned to receive PES (9.9% versus 22.9%, respectively; P=.002). Similarly, the rates of cardiac death, recurrent MI, and ST were significantly lower in those assigned to receive BAS (0.6% versus 5.1% [P=.02], 5.6% versus 15.6% [P=.002], and 0.0% versus 7.1% [P<.001], respectively). The rates of TLR, however, were statistically similar between the two stent groups (P=.22) (Figure 2A). On the other hand, among female patients, the primary composite endpoint of MACE was statistically similar between the two stent groups (P=.82). Similarly, the rates of cardiac death, recurrent MI, TLR, and ST were statistically similar between the two stent groups (P>.05 for all) (Figure 2B).
Stent-based analysis of the two gender subgroups. Among male patients, the primary composite endpoint of MACE was significantly lower in those assigned to receive BAS as compared with those assigned to receive PES (9.9% versus 22.9%, respectively; P=.002). Similarly, the rates of cardiac death, recurrent MI, and ST were significantly lower in those assigned to receive BAS (0.6% versus 5.1% [P=.02], 5.6% versus 15.6% [P=.002], and 0.0% versus 7.1% [P<.001], respectively). The rates of TLR, however, were statistically similar between the two stent groups (P=.22) (Figure 2A). On the other hand, among female patients, the primary composite endpoint of MACE was statistically similar between the two stent groups (P=.82). Similarly, the rates of cardiac death, recurrent MI, TLR, and ST were statistically similar between the two stent groups (P>.05 for all) (Figure 2B).
 Gender-based analysis of the two stent treatment arms. Among patients assigned to receive BAS, the primary composite endpoint of MACE was significantly higher in women as compared with men (23.1% versus 9.9%, respectively; P=.02) (Figure 3A). Similarly, TLR was significantly higher in women (19.2% versus 6.8%, respectively; P=.01). Both cardiac death and ST showed a trend to be higher in women as compared with men (3.8% versus 0.6% [P=.09] and 1.9% versus 0.0% [P=.08], respectively). Otherwise, the rates of recurrent MI and all-cause death were statistically matched between genders (P>.05 for both). On the other hand, among patients assigned to receive PES, all primary and secondary endpoints were statistically matched between genders (P>.05 for all) (Figure 3B).
Gender-based analysis of the two stent treatment arms. Among patients assigned to receive BAS, the primary composite endpoint of MACE was significantly higher in women as compared with men (23.1% versus 9.9%, respectively; P=.02) (Figure 3A). Similarly, TLR was significantly higher in women (19.2% versus 6.8%, respectively; P=.01). Both cardiac death and ST showed a trend to be higher in women as compared with men (3.8% versus 0.6% [P=.09] and 1.9% versus 0.0% [P=.08], respectively). Otherwise, the rates of recurrent MI and all-cause death were statistically matched between genders (P>.05 for both). On the other hand, among patients assigned to receive PES, all primary and secondary endpoints were statistically matched between genders (P>.05 for all) (Figure 3B).
 Independent predictors of MACE at 3-year follow-up. By multivariable logistic regression analysis, the independent predictors of MACE at 3-year follow-up were smaller stent diameter (P=.007; hazard ratio [HR], 2.05; 95% confidence interval [CI], 1.22-3.46), previous coronary bypass surgery (P=.007; HR, 3.22; 95% CI, 1.37-7.53), and assignment to the PES group (P=.003; HR, 2.06; 95% CI, 1.24-3.43).
Independent predictors of MACE at 3-year follow-up. By multivariable logistic regression analysis, the independent predictors of MACE at 3-year follow-up were smaller stent diameter (P=.007; hazard ratio [HR], 2.05; 95% confidence interval [CI], 1.22-3.46), previous coronary bypass surgery (P=.007; HR, 3.22; 95% CI, 1.37-7.53), and assignment to the PES group (P=.003; HR, 2.06; 95% CI, 1.24-3.43).
Discussion
Main findings. The current post hoc gender-based analysis of the TITAX-AMI trial demonstrated that the 3-year outcome of patients undergoing PCI for acute MI (irrespective of stent type) was slightly worse in female patients as compared with their male counterparts, as reflected by a trend toward a higher primary composite endpoint of MACE and a similar trend toward a higher TLR rate. Moreover, among female patients, those who received BAS had a 3-year outcome similar to those who received PES (P>.05 for all). However, among patients who received BAS, female patients had significantly higher rates of MACE and TLR, with trends toward higher rates of cardiac death and ST as compared with their male counterparts.
Cardiovascular disease in women. The rates of coronary artery disease in women increase by two- to three-fold after menopause.18 Moreover, it has recently been recognized that significant differences exist between men and women as regards coronary artery disease.19 Atypical presentation of angina and MI in women may eventually result in some delay in seeking medical care, a possible delay in diagnosis, and underuse of aggressive therapeutic approaches. Furthermore, women have been mostly under-represented in large clinical trials.19 Rather controversial results drawn from these trials underscoring a probably worse long-term clinical outcome in women undergoing PCI, as compared with men, may have, in a way, conditioned the referral process so that fewer women were subsequently referred to invasive procedures.20-22
Clinical outcome in females: TITAX-AMI trial. Despite the fact that females fared quite non-inferiorly as compared with their male counterparts as far as the long-term clinical outcome was concerned, all-cause death was significantly higher in the female subgroup. Although both genders were almost similar regarding the classic cardiovascular risk factors, substantially older age in the female subgroup may well have contributed to the overall mortality difference. Older age at presentation might in turn bring another variable into account: reduced creatinine clearance, a well-known predictor of worse outcome. Weighted evidence from literature similarly demonstrated higher-risk demographic and angiographic characteristics in females undergoing PCI, as compared with males.3,4,23,24 However, due to the retrospective nature of this post hoc analysis, some data relevant to outcome of PCI, such as body mass index, creatinine clearance, and time from symptom onset to intervention, have been overlooked.
Indeed, the trend toward a higher primary composite endpoint of MACE in the female subgroup was fundamentally driven by a trend toward higher TLR rates. Higher reintervention rates in female patients may be chiefly attributed to a significantly smaller reference vessel diameter in this subgroup, accounting for higher restenosis rates, ultimately causing further symptom-driven coronary angiography. Actually, within the female subgroup, TLR rate was numerically higher — albeit statistically insignificant — in patients assigned to receive BAS versus those assigned to receive PES (P=.29). Total MACE, however, and its two other components were better — again although statistically insignificant — in those who received BAS. It is noteworthy that among the male subgroup, total MACE, cardiac death, recurrent MI, and ST rates were all significantly better with BAS (P<.05 for all); TLR rate was better, but without meeting statistical significance.
In the trial arm that received BAS, the rate of total MACE was higher in females as compared with their male counterparts, obviously driven by a significantly higher rate of TLR. Once again, this may be viewed in light of the smaller-sized vessels in these patients, rather than any influence of gender, per se, on outcomes. Given the nearly similar gender-based rates of MACE and TLR in the arm that received PES, higher TLR in females who received BAS probably underlies the trend toward increased TLR in the female population as a whole. Albeit lower in the BAS group as compared with the PES group, the rate of cardiac death among patients who received BAS trended to be higher in females versus males, mostly driven by a trend toward a higher rate of ST in this particular subgroup. Although lower stent diameter, and quite possibly BAS undersizing, in the female subgroup might have contributed to the higher ST rates from a hypothetical perspective; the precise mechanisms underlying this surprising outcome are far from clear, and may leave a whole avenue for future research.
Independent predictors of outcome. Multivariable logistic regression analysis did not identify female gender among the independent predictors of MACE at 3-year follow-up. Instead, smaller stent diameter (again speaking of smaller coronary vessels), prior surgical revascularization, and allocation to PES independently predicted outcome. This makes a strong case for the theme that the divergence in outcome between the two gender subgroups most probably reflects differences in the nature of the underlying coronary disease, later presentation in life, and variance of comorbidities, rather than an influence of gender per se.
Study limitations. The TITAX-AMI trial was not designed to particularly explore gender-specific differences in outcome, whether as a pooled gender-based analysis or as regards gender and type of stent implanted. Furthermore, as already stated earlier, due to the retrospective nature of this post-hoc analysis, some data relevant to the outcome of PCI may have been missed. In addition, the trial may have been underpowered for specific subgroup analysis; therefore, any conclusions drawn from the analysis data should be taken with caution.
Conclusion
In the current post hoc gender-based analysis of the TITAX-AMI trial, the 3-year outcome of patients undergoing PCI for acute MI was slightly worse in female patients as compared with their male counterparts, as reflected by a trend toward a higher primary composite endpoint of MACE and a trend toward a higher TLR rate.
References
- Serruys PW, de Jaegere P, Kiemeneij F, et al. A comparison of balloon-expandable stent implantation with balloon angioplasty in patients with coronary artery disease. N Engl J Med. 1994;331(8):489-495.
- Fischman DL, Leon MB, Baim DS, et al. A randomized comparison of coronary stent placement and balloon angioplasty in the treatment of coronary artery disease. N Engl J Med. 1994;331(8):489-495.
- Lansky AJ, Costa RA, Mooney M; TAXUS-IV Investigators. Gender-based outcomes after paclitaxel-eluting stent implantation in patients with coronary artery disease. J Am Coll Cardiol. 2005;45(8):1180-1185.
- Kataoka Y, Yasuda S, Morii I, et al. Improved long-term prognosis of elderly women in the era of sirolimus-eluting stents. Circ J. 2009;73(7):1219-1227.
- Stone GW, Moses JW, Ellis SG, et al. Safety and efficacy of sirolimus- and paclitaxel-eluting coronary stents. N Engl J Med. 2007;356(10):998-1008.
- Morice MC, Serruys PW, Barragan P, et al. Long-term clinical outcomes with sirolimus-eluting coronary stents: five-year results of the RAVEL trial. J Am Coll Cardiol. 2007;50(14):1299-1304.
- Presbitero P, Belli G, Zavalloni D, et al. “Gender paradox” in outcome after percutaneous coronary intervention with paclitaxel-eluting stents. EuroIntervention. 2008;4(3):345-350.
- Spaulding C, Henry P, Teiger E, et al. Sirolimus-eluting versus uncoated stents in acute myocardial infarction. N Engl J Med. 2006;355(11):1093-1104.
- Laarman GJ, Suttorp MJ, Dirksen MT, et al. Paclitaxel-eluting versus uncoated stents in primary percutaneous coronary intervention. N Engl J Med. 2006;355(11):1105-1113.
- Menichelli M, Parma A, Pucci E, et al. Randomized trial of sirolimus-eluting stent versus bare-metal stent in acute myocardial infarction (SESAMI). J Am Coll Cardiol. 2007;49(19):1924-1930.
- Pasceri V, Patti G, Speciale G, et al. Meta-analysis of clinical trials on use of drug-eluting stents for treatment of acute myocardial infarction. Am Heart J. 2007;153(5):749-754.
- Stone GW, Lansky AJ, Pocock SJ, et al. Paclitaxel-eluting stent versus bare-metal stents in acute myocardial infarction. N Engl J Med. 2009;360(19):1946-1959.
- Iakovou I, Schmidt T, Bonizzoni E, et al. Incidence, predictors, and outcome of thrombosis after successful implantation of drug-eluting stents. JAMA. 2005;293(17):2126-2130.
- Karjalainen PP, Ylitalo A, Airaksinen KE. Real world experience with the TITAN stent: a 9-month follow-up report from the Titan PORI registry. Eurointervention. 2006;2(2):187-191.
- Mosseri M, Miller H, Tamari I, et al. The titanium-NO stent: results of a multicenter registry. Eurointervention. 2006;2(2):192-196.
- Karjalainen PP, Ylitalo A, Niemela M, et al. Two-year follow-up after percutaneous coronary intervention with titanium-nitride-oxide-coated stents versus paclitaxel-eluting stents in acute myocardial infarction. Ann Med. 2009;41(8):599-607.
- Karjalainen PP, Ylitalo A, Niemela M, et al. Titanium-nitride-oxide coated stents versus paclitaxel-eluting stents in acute myocardial infarction: a 12-months follow-up report from the TITAX-AMI trial. EuroIntervention. 2008;4(2):234-241.
- Thom T, Haase N, Rosamond W. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics — 2006 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2006;113(6):E85-E151.
- Mikhail GW. Coronary heart disease in women. Br Med J. 2005;331(7515):467-468.
- Ellis SG, Roubin GS, King SB 3rd, et al. Angiographic and clinical predictors of acute closure after native vessel coronary angioplasty. Circulation. 1988;77(2):372-379.
- Watanabe CT, Maynard C, Ritchie JL. Comparison of short-term outcomes following coronary artery stenting in men versus women. Am J Cardiol. 2001;88(8):848-852.
- Argulian E, Patel AD, Abramson JL, et al. Gender differences in short-term cardiovascular outcomes after percutaneous coronary interventions. Am J Cardiol. 2006;98(1):48-53.
- Lansky AJ, Ng VG, Mutlu H, et al. Gender-based evaluation of the XIENCE V everolimus-eluting coronary stent system: clinical and angiographic results from the SPIRIT III randomized trial. Catheter Cardiovasc Interv. 2009;74(5):719-727
- Seth A, Serruys PW, Lansky A, et al. A pooled gender based analysis comparing the XIENCE V everolimus-eluting stent and the TAXUS paclitaxel-eluting stent in male and female patients with coronary artery disease, results of the SPIRIT II and SPIRIT III studies: two-year analysis. EuroIntervention. 2010;5(7):788-794.
___________________________________________
From the 1Heart Center and Department of Internal Medicine, Kuopio University Hospital, Kuopio, Finland, 2Department of Cardiology, Satakunta Central Hospital, Pori, Finland,3Department of Internal Medicine, Division of Cardiology, University of Oulu, Oulu, Finland, 4Department of Medicine, Turku University Hospital, Turku, Finland, 5Department of Cardiology, Kokkola Central Hospital, Kokkola, Finland, and 6Department of Medicine, Jyväskylä Central Hospital, Jyväskylä, Finland.
Disclosure: The authors have completed and returned the ICMJE Form for Disclosure of Potential Conflicts of Interest. The authors report no conflicts of interest regarding the content herein.
Manuscript submitted October 13, 2011, provisional acceptance given November 3, 2011, final version accepted December 20, 2011.
Address for correspondence: Petri O. Tuomainen, MD, PhD, The University of Eastern Finland, P.O. Box 1627, FI-70211 Kuopio, Finland. Email: Petri.Tuomainen@uef.fi











