ADVERTISEMENT
Drug-Eluting Stenting of Saphenous Vein Graft Versus Native Coronary Artery Supplying the Same Myocardial Perfusion Territory: A Pilot Retrospective 3-Year Follow-up
Abstract: Background. In post-coronary artery bypass graft (CABG) patients undergoing drug-eluting stent implantation of either the saphenous vein graft (SVG) versus the native coronary artery supplying the same myocardial perfusion territory, which option confers better clinical outcomes when both lesions are technically feasible? Methods. From 2005 to 2008 at a single medical center, a total of 178 post-CABG patients (with 241 lesions) underwent PCI due to progressive SVG disease. Of them, 23 patients (with 29 lesions) had amenable disease for PCI in both the SVG and native coronary artery matching the same myocardial perfusion territory; chronic total occlusions were excluded. All patients included in the study were treated with drug-eluting stents. Sixteen patients (19 lesions) underwent PCI of the SVG, and 9 patients (10 lesions) underwent PCI in the native vessels. Results. Primary endpoints were in-hospital and 3-year rates of death, myocardial infarction (MI), target lesion revascularization (TLR), and target vessel revascularization (TVR). There were 2 in-hospital MIs in the SVG-treated group and 0 for the native vessel-treated group. The 3-year clinical follow-up showed 3 MIs, 2 TLRs, 4 TVRs, and 6 deaths in the SVG-treated group; only 1 MI occurred in the native-vessel treated group (P=.02). More PCIs of the SVG were performed than in the native coronary artery (19 vs 10 lesions). Conclusions. This small study suggests improved clinical outcomes with PCI of the native vessel, but a tendency of operators to choose PCI of the SVG instead. Large, prospective, multicenter, randomized clinical trials with long-term follow-up can validate the advantage of selecting PCI of the native vessel over the SVG when both options are available.
J INVASIVE CARDIOL 2012;24(10):516-520
Key words: degenerative saphenous vein graft
___________________________________________________
When encountering degenerative saphenous vein graft (SVG) disease, occasionally the bypassed native coronary artery lesion is also technically feasible for percutaneous coronary intervention (PCI). When both the SVG and native lesion are potentially treatable with PCI, which option tends to be chosen for treatment more frequently and which confers better clinical outcomes? Excluding the chronic total occlusions (CTO) in both the SVG and native coronary, this direct comparison has not been made.
Since bypassed vessels are likely to progress to more advanced disease,1 the encountered native coronary artery disease is likely to be of advanced lesion type for PCI based on the American College of Cardiology/American Heart Association (ACC/AHA) or the Society for Cardiovascular Angiography and Interventions (SCAI) classification systems, similar in category as the lesion type for all SVG stenoses. Intervention of bypassed native vessels, therefore, would likely result in similar success and complication rates.2 Clinical outcomes of SVG intervention are generally perceived as less favorable than PCI of native coronaries,3-5 even in the era of the drug-eluting stent (DES). Although direct randomized comparison of SVG vs native vessel PCI covering the same myocardial territory has not been performed, PCI of the native artery would appear to be the popular choice when both lesions are technically available for intervention.
A few small, non-randomized, and retrospective studies comparing PCI of SVG vs native coronary lesions showed no significant difference in the short- to long-term clinical outcomes.6-9 In these studies, however, the lesions for PCI were not necessarily matched for the same territory of myocardial ischemia. Also, CTOs, as a subset of unique lesions, were included in these studies. CTO carries lower procedural success rates dependent on the operator’s experience and skill sets.10,11 Along with varying outcomes after PCI of CTO vs non-CTO lesions in SVG vs native artery, these results should be reviewed with skepticism. The current study attempts to narrow the comparison of the choice between PCI of the SVG vs native coronary when both lesions are non-CTO and supply the same myocardial perfusion territory.
Methods
 Population. Demographic, clinical, and procedural data of patients undergoing PCI at our center were retrospectively mined from our regional ACC-National Cardiovascular Data Registry (NCDR) and electronic medical record (EMR) system (KP-HealthConnect). Between January 1, 2005 and December 31, 2008, a total of 178 patients underwent PCI (241 lesions) who had a previous history of coronary artery bypass surgery (CABG). After eliminating the left internal mammary artery bypass conduit, the corresponding left anterior descending (LAD) and CTO as the culprit lesion, 33 patients (with 39 lesions) had feasible lesions for PCI in both the SVG and the native coronary artery; hence, either can be the index lesion for PCI to restore adequate perfusion to the same myocardium at risk. Ten patients (with 10 lesions) were further excluded from the study due to the following reasons: lost to follow-up due to change of health plan and therefore no longer in the EMR system (5 patients), treatment with bare-metal stent (2 patients), treatment to both SVG and native artery to the same myocardial perfusion territories (2 patients), and treatment with balloon angioplasty only (1 patient). In the remaining study cohort, 23 patients (with 29 lesions) were included in the final analyses. Sixteen of these patients (19 lesions) underwent PCI of the SVG, and 9 patients (with 10 lesions) underwent PCI of the native coronary artery (Table 1). Two patients had PCI in both SVG and native vessel but of different myocardial perfusion territories, and were tracked accordingly to the sites of treatment. The feasibility for PCI of the lesions was reviewed and agreed upon by 3 experienced interventionalists. Patients who were treated with redo-CABG or medical management were not included in the ACC-NCDR and were not included in the initial data mining of this study.
Population. Demographic, clinical, and procedural data of patients undergoing PCI at our center were retrospectively mined from our regional ACC-National Cardiovascular Data Registry (NCDR) and electronic medical record (EMR) system (KP-HealthConnect). Between January 1, 2005 and December 31, 2008, a total of 178 patients underwent PCI (241 lesions) who had a previous history of coronary artery bypass surgery (CABG). After eliminating the left internal mammary artery bypass conduit, the corresponding left anterior descending (LAD) and CTO as the culprit lesion, 33 patients (with 39 lesions) had feasible lesions for PCI in both the SVG and the native coronary artery; hence, either can be the index lesion for PCI to restore adequate perfusion to the same myocardium at risk. Ten patients (with 10 lesions) were further excluded from the study due to the following reasons: lost to follow-up due to change of health plan and therefore no longer in the EMR system (5 patients), treatment with bare-metal stent (2 patients), treatment to both SVG and native artery to the same myocardial perfusion territories (2 patients), and treatment with balloon angioplasty only (1 patient). In the remaining study cohort, 23 patients (with 29 lesions) were included in the final analyses. Sixteen of these patients (19 lesions) underwent PCI of the SVG, and 9 patients (with 10 lesions) underwent PCI of the native coronary artery (Table 1). Two patients had PCI in both SVG and native vessel but of different myocardial perfusion territories, and were tracked accordingly to the sites of treatment. The feasibility for PCI of the lesions was reviewed and agreed upon by 3 experienced interventionalists. Patients who were treated with redo-CABG or medical management were not included in the ACC-NCDR and were not included in the initial data mining of this study.
Endpoints. The primary endpoints were the occurrences of myocardial infarction (MI), target vessel revascularization (TVR), target lesion revascularization (TLR), death, and composite major adverse cardiac events (MACE) including all causes of death, nonfatal MI, and/or repeat revascularization periprocedurally and during the 3-year follow-up period.
Definitions. Procedural success was defined as the ability for stent deployment with a residual stenosis of <20% and no in-hospital MACE. MI was defined as either 3x CK-MB elevation from baseline and/or troponin-I >0.2 ng/mL. TLR was defined as revascularization performed on the treated lesion, and TVR was defined as revascularization on the treated vessel.
Interventional procedure. The PCI procedures were performed according to standard preparation and technique via femoral approach. Procedural and technical preferences were left to the discretion of the operators. During the procedure, all patients received adequate heparin administration to achieve an activated clotting time of >200 or 250 seconds depending on the use of intravenous glycoprotein IIb/IIIa platelet inhibitor. All lesions except for 3 SVG lesions were treated with paclitaxel-eluting stents; 2 sirolimus-eluting stents and 1 everolimus-eluting stent were placed in the remaining 3 SVG lesions. The patients were loaded with clopidogrel plus the continuation of daily 75 mg clopidogrel and 325 mg aspirin for at least 1 year after the procedure.
Follow-up. Because of a relatively isolated community and an integrated health-care system, all of the patients’ clinical visits and hospitalizations were charted in the regional EMR. Data regarding all endpoints, including MI, TLR, TVR, death, and MACE, were searched and collected from the EMR on all of the study patients. Angiographic follow-up was performed on patients with recurrent angina and/or MI. Repeat revascularization was performed when clinically appropriate.
Statistical analysis. The Fisher’s exact test was used in the comparison of 2 classes between the 2 categories in all 2 x 2 tables, since the counts in some cells of the 2 x 2 table are smaller than 5. The z tests from the least square means of logistic model was used in outcome variables having more than 2 classes. The pooled t-test was used when the means between the 2 categories were compared and the variances in the 2 categories were equal. If the variances were unequal, the Satterthwaite t-test was used. Continuous variables were expressed as mean ± standard deviation; categorical variables were expressed as counts (n) and percentages (%). A P-value of >.05 was considered statistically insignificant (NS).
Results
Baseline clinical variables. The baseline clinical characteristics of the patient population are shown in Table 1. Comparing PCI of the native artery group versus PCI of the SVG group, there was no difference in age, sex, cardiac risk factors, prior cardiac history (prior MI, PCI, CABG), age of the bypass grafts, LVEF at the index PCI, medication use (aspirin, beta-blocker, statin), indication for PCI (unstable angina, ischemia on stress test, MI), or extent of CAD.
 Baseline angiographic and procedural variables (Table 2). Comparing the PCI of native artery group versus SVG group, there was no statistical difference in the coronary artery distribution of the intervened vessels. No statistical difference was present in the ACC/AHA lesion classification between the 2 groups; nearly all of the lesions in the study were in the “high-risk” or type C category. The Filterwire (Boston Scientific) was the only embolic protection device available in the laboratory during the study period. Only 1/19 SVG PCIs was performed with the Filterwire; most of them were not technically feasible for distal device protection due to small-caliber vessels and inadequate distal landing zones (10/18). The rest were deemed lower-risk lesions by the operators: (4/18) in-stent restenotic and (4/18) aorto-ostial lesions (see discussion). Nonetheless, TIMI 3 flow was obtained in all of the post-PCI angiograms, with 100% angiographic success rates in both groups.
Baseline angiographic and procedural variables (Table 2). Comparing the PCI of native artery group versus SVG group, there was no statistical difference in the coronary artery distribution of the intervened vessels. No statistical difference was present in the ACC/AHA lesion classification between the 2 groups; nearly all of the lesions in the study were in the “high-risk” or type C category. The Filterwire (Boston Scientific) was the only embolic protection device available in the laboratory during the study period. Only 1/19 SVG PCIs was performed with the Filterwire; most of them were not technically feasible for distal device protection due to small-caliber vessels and inadequate distal landing zones (10/18). The rest were deemed lower-risk lesions by the operators: (4/18) in-stent restenotic and (4/18) aorto-ostial lesions (see discussion). Nonetheless, TIMI 3 flow was obtained in all of the post-PCI angiograms, with 100% angiographic success rates in both groups.
The findings, however, showed a significant difference in the dimensions and number of stents used between the 2 groups. Comparing the native artery group to the SVG group, there was a significantly higher number of stents used per lesion, and longer length of the stented segments. The final stent diameters were larger in the SVG group, probably reflecting the natural size difference between vein grafts and native coronary arteries.
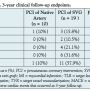
 Follow-up clinical outcomes: procedural, in-hospital, and at 3 years. Table 3 shows the procedural and in-hospital endpoint results. In the SVG group, there was 1 prophylactic periprocedural intra-aortic balloon pump (IABP) used and 2 in-hospital MIs; no events occurred in the native artery group. Although all 3 events occurred in the SVG group, they did not comprise a statistical difference, as shown. Table 4 shows the 3-year clinical follow-up endpoint results. In the SVG group, there were 3 MIs, 2 TLRs, 4 TVRs, and 6 deaths; there was 1 MI in the native artery group. The composite MACE rate demonstrated a statistical significance with a P-value of .02 favoring PCI of the native artery group.
Follow-up clinical outcomes: procedural, in-hospital, and at 3 years. Table 3 shows the procedural and in-hospital endpoint results. In the SVG group, there was 1 prophylactic periprocedural intra-aortic balloon pump (IABP) used and 2 in-hospital MIs; no events occurred in the native artery group. Although all 3 events occurred in the SVG group, they did not comprise a statistical difference, as shown. Table 4 shows the 3-year clinical follow-up endpoint results. In the SVG group, there were 3 MIs, 2 TLRs, 4 TVRs, and 6 deaths; there was 1 MI in the native artery group. The composite MACE rate demonstrated a statistical significance with a P-value of .02 favoring PCI of the native artery group.
Discussion
 The study retrospectively reviewed the clinical outcomes over 3 years of post-CABG patients receiving PCI to either the SVG or the corresponding native coronary artery supplying the same myocardial perfusion territory, when both lesions were technically amenable to PCI. After excluding technical unfeasibility, LIMA-related lesions, CTO in either SVG or native artery, and those lost to follow-up, the portion of patients who met the study criteria of DES treatment to either the SVG versus native artery was rather small, comprising approximately 12% of the overall patient pool of PCI after CABG. In this small group of cohorts, the baseline clinical, angiographic, and procedural characteristics were well-matched, with no significant difference between the PCI to native artery versus SVG groups. The finding was also insignificant for in-hospital MACE, although 2 in-hospital MIs along with 1 IABP placement occurred in the SVG versus 0 events in the native artery group. Most importantly, the results were significant for composite MACE at 3-year follow-up in the SVG group, compared to the native artery group.
The study retrospectively reviewed the clinical outcomes over 3 years of post-CABG patients receiving PCI to either the SVG or the corresponding native coronary artery supplying the same myocardial perfusion territory, when both lesions were technically amenable to PCI. After excluding technical unfeasibility, LIMA-related lesions, CTO in either SVG or native artery, and those lost to follow-up, the portion of patients who met the study criteria of DES treatment to either the SVG versus native artery was rather small, comprising approximately 12% of the overall patient pool of PCI after CABG. In this small group of cohorts, the baseline clinical, angiographic, and procedural characteristics were well-matched, with no significant difference between the PCI to native artery versus SVG groups. The finding was also insignificant for in-hospital MACE, although 2 in-hospital MIs along with 1 IABP placement occurred in the SVG versus 0 events in the native artery group. Most importantly, the results were significant for composite MACE at 3-year follow-up in the SVG group, compared to the native artery group.
Studies have shown that PCI treatment of SVGs generally carries worsened outcomes compared to native coronary arteries in various clinical settings. In ST-segment elevation MI, treatment with primary PCI due to SVG occlusion has poor acute procedural results, frequent late reocclusion, and very high late mortality as compared to primary PCI for native vessel occlusions.12 In a pooled analysis of 5 randomized trials of PCI using glycoprotein IIb/IIIa platelet inhibitor, Roffi et al demonstrated that PCI of a bypass graft was identified as an independent predictor of death, myocardial infarction, or revascularization at 6 months.13 When compared to bare-metal stent (BMS), treatment of SVG with DES appears to be favorable in the short-term;14-17 however, the initial benefits may wane in longer-term follow-up close to 3 years.18,19 On the contrary, the improvement of outcomes of DES versus BMS holds up in long-term follow-up studies in the treatment of native coronary arteries.20-22 The long-term follow-up of our current study highlighted these differences in a direct comparison of PCI with DES in SVG versus native artery matching the same myocardial territory.
Although embolic protection devices (EPD) are recommended in SVG PCI when technically feasible,23 real-world usage has been significantly limited due to anatomic unsuitability and operator discretion.24-26 In an analysis of 19,546 patients undergoing SVG PCI in the ACC-NCDR Data Registry, Mehta et al found only 22% received an EPD.24 Matar et al further determined, in a study of 2541 patients, that only 14.6% of SVG PCIs were technically feasible for the filter-based EPD. The most common reasons for EPD ineligibility were small vessel size and lack of adequate landing zone for the filter-basket,25,26 as observed in our study. During the study period of these studies as well as the current study, only the large-vessel Filterwire was available, further limiting the applicability of EPD at that time. Other studies have found low-risk SVG lesions for distal embolization during PCI including in-stent restenotic27,28 and aorto-ostial lesions.29,30 These additional factors may further contribute to the decline in the real-world utilization of EPD in SVG PCI.25,26,31 In the current study, 10/19 (53%) of the SVG lesions were unprotectable due to small vessel size and poor distal landing zone; the remaining unprotected lesions were in-stent restenotic and ostial SVG lesions. Utilization of EPD in this study reflects the real-world practice and is a valid factor when comparing to PCI of the native coronaries when the option is available.
Other findings of this study further suggest the benefits of PCI of the native artery over the SVG when given the choice. In the post-PCI stent analysis, the findings were significant for shorter stented segments, fewer stents per lesion, and larger final stent diameter in the SVG group compared to the native artery group. Despite having these features that should favor the SVG group in clinical outcomes, the SVG group had a significant composite MACE at 3-year follow-up. Yet, the study also found that more SVG lesions were chosen as the PCI target by the operators in a 2:1 margin (66% for SVG; 34% for native artery). Perhaps the occasional focal SVG lesion may be visually more enticing as a PCI target; however, the unrevascularized native vessel will likely progress toward CTO, which can pose a difficult challenge for future PCI. As such, if native coronary PCI does indeed provide better clinical outcomes over that of SVG, it should be chosen as PCI target whenever feasible. In addition, the availability of longer stent lengths up to 38 mm should further encourage native vessel PCI in the case of long-lesion PCI, lowered restenosis rate and lowered cost of fewer stents used.
Study limitations. This study was limited by its small sample size, retrospective, single-center and non-randomized nature. By using strict criteria for PCI feasibility of lesions, many borderline cases were excluded. The remaining patients included in the analyses were a small group; however, the findings serve as pilot results for future confirmatory studies.
Conclusion
When given the choice of PCI of the SVG versus the native coronary artery supplying the same myocardial perfusion territory, this small study suggests improved clinical outcomes with PCI of the native vessel. Tendency of operators, however, appears to favor PCI of the SVG instead. If indeed better, operators should be encouraged to choose native coronary lesions as primary PCI targets when technically feasible. Large, prospective, multicenter, randomized clinical trials with long-term follow-up can validate the advantage of selecting PCI of the native vessel over the SVG when both options are available.
References
- Guthaner DF, Robert EW, Alderman EL, Wexler L. Long-term serial angiographic studies after coronary artery bypass surgery. Circulation. 1979;60(2):250-259.
- Krone RJ, Shaw RE, Klein LW, et al; for the ACC-National Cardiovascular Data Registry. Evaluation of the American College of Cardiology/American Heart Association and the Society for Coronary Angiography and Interventions lesion classification system in the current “stent era” of coronary interventions (from the ACC-National Cardiovascular Data Registry). Am J Cardiol. 2003;92(4):389-394.
- Ge L, Iakovou I, Sangiorgi GM, et al. Treatment of saphenous vein graft lesions with drug-eluting stents. J Am Coll Cardiol. 2005;45(7):989-994.
- Moses JW, Leon MB, Popma JJ, et al. Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery. N Engl J Med. 2003;349(14):1315-1323.
- Stone GW, Ellis SG, Cox DA, et al. A polymer-based, Paclitaxel-eluting stent in patients with coronary artery disease. N Engl J Med 2004;350(3):221-231.
- Varghese I, Samuel J, Banerjee S, Brilakis ES. Comparison of percutaneous coronary intervention in native coronary arteries vs bypass grafts in patients with prior coronary artery bypass graft surgery. Cardiovasc Revasc Med. 2009;10(2):103-109.
- Garcia-Tejada J, Velazquez M, Hernandez F, et al. Percutaneous revascularization of grafts versus native coronary arteries in postcoronary artery bypass graft patients. Angiology. 2009;60(1):60-66.
- D’Ascenzo F, Gonella A, Longo G, et al. Short and long-term outcomes of percutaneous revascularization in patients with prior coronary artery bypass graft. Minerva Cardioangiologica. 2010;58(3):291-299.
- Meliga E, Garcia-Garcia HM, Kukreja N, et al. Chronic total occlusion treatment in post-CABG patients: saphenous vein graft versus native vessel recanalization — long-term follow-up in the drug-eluting stent era. Catheter Cardiovasc Interv. 2007;70(1):21-25.
- Grantham JA, Marso SP, Spertus J, House J, Holmes DR Jr, Rutherford BD. Chronic total occlusion angioplasty in the United States. JACC Cardiovasc Interv. 2009;2(6):479-486.
- Thompson CA, Jayne JE, Robb JF, et al. Retrograde techniques and the impact of operator volume on percutaneous intervention for coronary chronic total occlusions an early US experience. JACC Cardiovasc Interv. 2009;2(9):834-842.
- Brodie BR, VerSteeg DS, Brodie MM, et al. Poor long-term patient and graft survival after primary percutaneous coronary intervention for acute myocardial infarction due to saphenous vein graft occlusion. Catheter Cardiovasc Interv. 2005;65(4):504-509.
- Roffi M, Mukherjee D, Chew DP, et al. Lack of benefit from intravenous platelet glycoprotein IIb/IIIa receptor inhibition as adjunctive treatment for percutaneous interventions of aortocoronary bypass grafts: a pooled analysis of five randomized clinical trials. Circulation. 2002;106(24):3063-3067.
- Minutello RM, Bhagan S, Sharma A, et al. Long-term clinical benefit of sirolimus-eluting stents compared to bare metal stents in the treatment of saphenous vein graft disease. J Interv Cardiol. 2007;20(6):458-465.
- Lee MS, Shah AP, Aragon J, et al. Drug-eluting stenting is superior to bare metal stenting in saphenous vein grafts. Catheter Cardiovasc Interv. 2005;66(4):507-511.
- Vermeersch P, Agostoni P, Verheye S, et al. Randomized double-blind comparison of sirolimus-eluting stent versus bare-metal stent implantation in diseased saphenous vein grafts: six-month angiographic, intravascular ultrasound, and clinical follow-up of the RRISC Trial. J Am Coll Cardiol. 2006;48(12):2423-2431.
- Mehilli J, Pache J, Abdel-Wahab M, et al; for the “Is Drug-Eluting-Stenting Associated with Improved Results in Coronary Artery Bypass Grafts?” (ISAR-CABG) investigators. Drug-eluting versus bare-metal stents in saphenous vein graft lesions (ISAR-CABG): a randomized controlled superiority trial. Lancet. 2011;378(9796):1071-1078.
- Vermeersch P, Agostoni P, Verheye S, et al; DELAYED RRISC (Death and Events at Long-term follow-up AnalYsis: Extended Duration of the Reduction of Restenosis in Saphenous vein grafts with Cypher stent) investigators. Increased late mortality after sirolimus-eluting stents versus bare-metal stents in diseased saphenous vein grafts: results from the randomized DELAYED RRISC trial. J Am Coll Cardiol. 2007;50(3):261-267.
- Bansal D, Muppidi R, Singla S, et al. Percutaneous intervention on the saphenous vein bypass grafts — long-term outcomes. Catheter Cardiovasc Interv. 2008;71(1):58-61.
- Harjai KJ, Sattur S, Orshaw P, Boura J. Long-term safety and effectiveness of drug-eluting stents compared to bare metal stents following successful PCI in non-ST elevation myocardial infarction: findings from the Guthrie Health Off-Label StenT (GHOST) registry. J Interv Cardiol. 2012;25(1):28-36.
- Puymirat E, Mangiacapra F, Peace A, et al. Long-term clinical outcome in patients with small vessel disease treated with drug-eluting versus bare metal stenting. Am Heart J. 2011;162(5):907-913.
- Ramanath VS, Brown JR, Malenka DJ, et al; Dartmouth Dynamic Registry Investigators. Outcomes of diabetics receiving bare-metal stents versus drug-eluting stents. Catheter Cardiovasc Interv. 2010;76(4):473-481.
- Levine GN, Bates ER, Blankenship JC, et al. 2011 ACCF/AHA/SCAI guidelines for percutaneous coronary intervention. Circulation. 2011;124:E574-E651.
- Mehta SK, Frutkin AD, Milford-Beland S, et al. American College of Cardiology-National Cardiovascular Data Registry. Utilization of distal embolic protection in saphenous vein graft interventions (an analysis of 19,546 patients in the American College of Cardiology-National Cardiovascular Data Registry). Am J Cardiol. 2007;100(7):1114-1118.
- Matar FA, Smith K, Rossi P, et al. Limitations of embolic protection in saphenous vein graft intervention: insights from 202 consecutive patients. J Interv Cardiol. 2009;22(3):240-246.
- Badhey N, Lichtenwalter C, de Lemos JA, et al. Contemporary use of embolic protection devices in saphenous vein graft interventions: Insights from the stenting of saphenous vein grafts trial. Catheter Cardiovasc Interv. 2010;76(2):263-269.
- Assali AR, Sdringola S, Moustapha A, et al. Percutaneous intervention in saphenous venous grafts: in-stent restenosis lesions are safer than de novo lesions. J Invasive Cardiol. 2001;13(6):446-450.
- Cicek D, Doven O, Pekdemir H, et al. Procedural results and distal embolization after saphenous vein graft stenting and angioplasty for in-stent restenosis of grafts. Jpn Heart J. 2004;45(4):561-571.
- Sano K, Mintz GS, Carlier SG, et al. Intravascular ultrasonic differences between aorto-ostial and shaft narrowing in saphenous veins used as aortocoronary bypass grafts. Am J Cardiol. 2006;97(10):1463-1466.
- Hong YJ, Jeong MH, Ahn Y, et al. Impact of lesion location on intravascular ultrasound findings and short-term and five-year long-term clinical outcome after percutaneous coronary intervention for saphenous vein graft lesions. Int J Cardiol. 2011 Dec 20 (Epub ahead of print).
- Lavi S, Ivanov J, Appleby CE, et al. Selective use of embolic protection devices during saphenous vein grafts interventions: a single-center experience. Catheter Cardiovasc Interv. 2010;75(7):1037-1044.
___________________________________________________
From the 1Division of Cardiology, Hawaii Permanente Medical Group, Honolulu, Hawaii, 2the Division of Cardiology, Northern California Permanente Medical Group, Santa Clara, California, and 3the Division of Cardiology, Southern California Permanente Medical Group, San Diego, California.
Disclosure: The authors have completed and returned the ICMJE Form for Disclosure of Potential Conflicts of Interest. The authors report no conflicts of interest regarding the content herein.
Manuscript submitted April 20, 2012 and accepted May 22, 2012.
Address for correspondence: Paul C. Ho, MD, MSc, FACC, FSCAI, Chief, Division of Cardiology, Hawaii Region Kaiser Permanente, 3288 Moanalua Road, Honolulu, HI 96819. Email: paul.c.ho@kp.org
















