ADVERTISEMENT
Irreversible Delayed Complete Heart Block Secondary to Jailed First Septal Perforator Following PCI of the Left Anterior Descending Coronary Artery
ABSTRACT: Permanent complete heart block (CHB) secondary to the loss of first septal perforator after percutaneous coronary intervention (PCI) of the left descending artery (LAD) is an extremely rare complication. We describe a case report where a patient underwent PCI of proximal LAD, complicated by loss of first septal perforator, septal infarction, and bifasicular block, which progressed to symptomatic delayed CHB. One week later, the patient required implantation of a permanent pacemaker following failure to wean off the transvenous temporary pacing maker.
J INVASIVE CARDIOL 2012;24:E13-E15
Key words: PCI of LAD, loss of first septal perforator, complete heart block
___________________________________________
Complete heart block (CHB) after jailing of first septal perforator following percutaneous coronary intervention (PCI) of the left anterior descending artery (LAD), although uncommon, is usually transient, occurring during the procedure and resolving within 72 hours. ECG changes of ischemia, conduction disturbance, ventricular arrhythmias, and acute myocardial infarction had been described following occlusion of septal perforators. Our review of English literature revealed only 3 published cases of delayed CHB secondary to jailing of the first septal perforator with only one case requiring implantation of a permanent pacemaker (PPM).1-3 To our knowledge, this is the second case report of delayed CHB that required permanent pacemaker implantation secondary to loss of first septal perforator following PCI to proximal LAD.
Our case highlights the importance of longer than usual post-procedural patient observation and ECG monitoring after jailing of first septal perforator and considering necessary steps if required.
 Case Report. A 75-year-old female patient with hypertension and previous history of a transient ischemic attack presented to our emergency department with 3 days history of epigastric and retrosternal chest pain, and recent onset of shortness of breath on exertion. Pain was not radiating and was not associated with any sweating or palpitation. On examination, patient was hemodynamically stable with no signs of heart failure and her systemic examination was unremarkable. ECG showed heart rate of 77 BPM and biphasic T waves from V1-V6. Her Troponin T was elevated (0.59 ng/mL). She was admitted with the diagnosis of non ST elevation myocardial infarction (NSTEMI) and went for coronary angiogram within 24 H based on decision for an early invasive therapy.
Case Report. A 75-year-old female patient with hypertension and previous history of a transient ischemic attack presented to our emergency department with 3 days history of epigastric and retrosternal chest pain, and recent onset of shortness of breath on exertion. Pain was not radiating and was not associated with any sweating or palpitation. On examination, patient was hemodynamically stable with no signs of heart failure and her systemic examination was unremarkable. ECG showed heart rate of 77 BPM and biphasic T waves from V1-V6. Her Troponin T was elevated (0.59 ng/mL). She was admitted with the diagnosis of non ST elevation myocardial infarction (NSTEMI) and went for coronary angiogram within 24 H based on decision for an early invasive therapy.
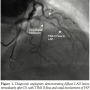
 The patient’s angiogram revealed a diffuse LAD lesion immediately after the first diagonal (D1) with subtotal occlusion and TIMI II flow distal to lesion. The lesion also involved the ostium of the second diagonal (D2) and first septal perforator (Figure 1). Distal left circumflex coronary had a borderline lesion (60%-70%) and right coronary artery was non-dominant and small caliber. During PCI, the LAD lesion was crossed with a Fielder XT Coronary Guidewire (Abbott Vascular) and then exchanged with a Runthrough NS Guidewire (Terumo) using a FineCross Microcatheter (Terumo). A Whisper Guidewire (Guidant Corp.) was steered to the first septal perforator (FSP). The LAD lesion was dilated with a Voyager RX 2.2 x 15 mm semi-compliant balloon (Abbott) and a Sapphire NC 2.5 x 20 mm non-compliant balloon (OrbusNeich). The ostial FSP lesion was dilated with a Voyager RX 2.2 x 15 mm semi-compliant balloon. Following stent deployment (bare metal stent 3.0 x 28 mm) at LAD, there was loss of the FSP and D2, presumed to be due to plaque shift or “snow plow” effect during stent deployment (Figure 2). The flow to LAD was TIMI III with no residual stenosis, dissection, or thrombus. The patient developed transient chest pain with ST elevation following loss of the FSP and D2. Attempts to wire the FSP through the LAD stent struts remained unsuccessful.
The patient’s angiogram revealed a diffuse LAD lesion immediately after the first diagonal (D1) with subtotal occlusion and TIMI II flow distal to lesion. The lesion also involved the ostium of the second diagonal (D2) and first septal perforator (Figure 1). Distal left circumflex coronary had a borderline lesion (60%-70%) and right coronary artery was non-dominant and small caliber. During PCI, the LAD lesion was crossed with a Fielder XT Coronary Guidewire (Abbott Vascular) and then exchanged with a Runthrough NS Guidewire (Terumo) using a FineCross Microcatheter (Terumo). A Whisper Guidewire (Guidant Corp.) was steered to the first septal perforator (FSP). The LAD lesion was dilated with a Voyager RX 2.2 x 15 mm semi-compliant balloon (Abbott) and a Sapphire NC 2.5 x 20 mm non-compliant balloon (OrbusNeich). The ostial FSP lesion was dilated with a Voyager RX 2.2 x 15 mm semi-compliant balloon. Following stent deployment (bare metal stent 3.0 x 28 mm) at LAD, there was loss of the FSP and D2, presumed to be due to plaque shift or “snow plow” effect during stent deployment (Figure 2). The flow to LAD was TIMI III with no residual stenosis, dissection, or thrombus. The patient developed transient chest pain with ST elevation following loss of the FSP and D2. Attempts to wire the FSP through the LAD stent struts remained unsuccessful.
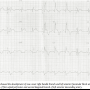 Post PCI, the patient was observed in the coronary care unit and remained pain free with no acute hemodynamic compromise. An ECG showed a new onset right bundle branch block (RBBB), left anterior fascicular block (LAFB), ST elevation at I, aVL, V2-V4 consistent with loss of FSP and D2 (Figure 3).
Post PCI, the patient was observed in the coronary care unit and remained pain free with no acute hemodynamic compromise. An ECG showed a new onset right bundle branch block (RBBB), left anterior fascicular block (LAFB), ST elevation at I, aVL, V2-V4 consistent with loss of FSP and D2 (Figure 3).
More than 48 H post PCI, the patient developed an episode of unresponsiveness, which was attributed to hypotension from CHB with a heart rate of 31 BPM. She was electively intubated for airway protection and required inotropes for blood pressure support. An emergency repeat angiogram showed patent stent of LAD with TIMI III flow. There was improvement in FSP blood flow but no contrast filling of its branches fanning out from the distal end on diagnostic angiogram. She had a temporary transvenous pacemaker and intra-aortic balloon pump (IABP) insertion for hemodynamic support. Subsequently, the patient improved hemodynamically and weaned off IABP and mechanical ventilatory support. However, she was fully pacemaker dependent and unable to wean off the temporary transvenous pacemaker after 7 days, resulting in implantation of PPM.
Discussion. PCI is a novel approach for the treatment of critical coronary artery stenosis. Side branch occlusion is not an uncommon complication of PCI occurring in up to 18% of patients.4 Atheromatous plaque shift or the “snow plow” effect into the side branch is reported to be the mechanism responsible.5,6 Documentation of transient CHB due to jailing of the first septal perforator during PCI of LAD is not infrequent. However, occurrence of irreversible or delayed CHB following occlusion of FSP after PCI of LAD is an extremely rare complication. Our English literature review found only 3 published reports of delayed CHB following jailing of FSP with only one case of permanent delayed CHB requiring implantation of PPM.1 In the case reported by Pillai et al, spontaneous reversion to sinus rhythm with normal PR interval and QRS complexes was observed 12 hours after the onset of delayed CHB. Kireyev et al performed successful intervention of FSP in their patient with delayed CHB with a gradual return of AV conduction via the left bundle branch within 48 hours. Although the septal artery angioplasty is relatively safe and not associated with aortic dissection, myocardial infarction (MI), or death, we decided not to reattempt wiring of FSP as CHB associated with anterior MI is usually considered to be irreversible.
The first septal perforator supplies the superior and anterior portion of the interventricular septum; its occlusion following PCI of LAD can cause infarction, conduction disturbances arising to symptoms of angina, arrhythmia, and heart failure. ECG changes vary from sinus bradycardia, first-degree AV block, new onset RBBB, and LAFB to polymorphic ventricular tachycardia, complicating acute MI.1-3,7 In our patient, development of new onset bifasicular block (RBBB associated with LAFB) was a warning sign that the blood supply of her conduction system compromised. These kinds of patients are one step away from CHB. The delay in developing the CHB after occlusion of the first septal perforator was consistent with the fact that the damage to the conduction system caused by hypoperfusion and necrosis.
In our patient, development of CHB was preceded by new onset RBBB, a finding consistent with all 3 previous published reports, however, the timing of onset varied significantly.1-3 RBBB may occur immediately post-procedure, as in our patient, or as late as 48 H, making it less clear whether asymptomatic patients with or without new onset RBBB or pre-existing conduction abnormalities should be considered for longer hospital stay as compared to routine day care admission. Additionally, current guidelines don’t recommend the routine insertion of prophylactic pacing system in asymptomatic patients with bifasicular block complicating anterior MI. In our patient, we decided not to do prophylactic transvenous pacing and opted for continuously monitoring by means of telemetry. After more than 48 H our patient progressed to CHB with hemodynamic compromise and required temporary transvenous pacing with subsequent PPM implantation 1 week later due to inability to wean off temporary pacing wire.
Conclusion. We present an interesting and rare case of delayed CHB due to jailing of FSP following PCI to LAD. FSP occlusion may have significant clinical consequences in certain subgroups of patients. Extension of hospital stay for continuous ECG monitoring or prophylactic temporary transvenous pacing with new onset bifasicular block may be considered in such patients if institutional facilities are not available for rapid pacemaker insertion.
References
- Nee LM, Guttormsen B, Gimelli G. Delayed complete heart block secondary to jailed first septal perforator. J Invasive Cardiol. 2007;19(11):E338-339.
- Kireyev D, Page B, Young HG. Septal infarction and complete heart block following percutaneous coronary intervention of the left anterior descending coronary artery. J Invasive Cardiol. 2009;21(3):E48-50.
- Pillai RV, Daniel R, Joseph DJ. Complete heart block following occlusion of the first septal perforator after coronary stenting. Indian Heart J. 2005;57(6):728-730.
- Meier B, Gruentzig AR, King SB 3rd, et al. Risk of side branch occlusion during coronary angioplasty. Am J Cardiol. 1984;53(1):10-14.
- Cho GY, Lee CW, Hong MK, Kim JJ, Park SW, Park SJ. Side-branch occlusion after rotational atherectomy of in-stent restenosis: incidence, predictors, and clinical significance. Catheter Cardiovasc Interv. 2000;50 (4):406-410.
- Aliabadi D, Tilli FV, Bowers TR, et al. Incidence and angiographic predictors of side branch occlusion following high-pressure intracoronary stenting. Am J Cardiol. 1997;80(8):994-997.
- Furgerson JL, Sample SA, Gilman JK, Carlson TA. Complete heart block and polymorphic ventricular tachycardia complicating myocardial infarction after occlusion of the first septal perforator with coronary stenting. Cathet Cardiovasc Diagn. 1998;44(4):434-437.
___________________________________________
From the Cardiology Unit, Department of Medicine, University Malaya Medical Centre, 50603 Kuala Lumpur, Malaysia.
Disclosure: The authors have completed and returned the ICMJE Form for Disclosure of Potential Conflicts of Interest. The authors report no conflicts of interest regarding the content herein.
Manuscript submitted on June 16, 2011 and accepted July 27, 2011.
Address for correspondence: M. Athar Sadiq, Senior Lecturer, Cardiology Unit, Department of Medicine, University Malaya Medical Centre, 50603 Kuala Lumpur, Malaysia. Email: matharsadiq@gmail.com
















