ADVERTISEMENT
Late Stent Thrombosis of a Second-Generation Drug-Eluting Stent
ABSTRACT: A 62-year-old male patient presented with acute non-ST-elevation myocardial infarction. He underwent successful percutaneous coronary intervention with implantation of an everolimus-eluting stent in the left anterior descending coronary artery. Six months later, he discontinued clopidogrel. Two weeks later, he presented with unstable angina. Despite the unremarkable electrocardiography, cardiac biomarkers, and coronary angiography, optical coherence tomography revealed a thrombus extending throughout the stent, with uncovered and malapposed stent struts in its proximal part. Thrombectomy was performed. The patient was discharged on dual antiplatelet therapy. Eight months later, the follow-up coronary angiography reassured a patent stent with adequate flow and no evidence of thrombi.
J INVASIVE CARDIOL 2012;24(10):E225-E227
Key words: optical coherence tomography, stent thrombosis, drug-eluting stents
_________________________________________________________
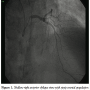 Case Report. A 62-year-old male patient presented to our emergency department in June 2009 with persistent ischemic-type chest pain for 3 H. He was an insulin-dependent diabetic, hypertensive, dyslipidemic, with chronic renal impairment. Electrocardiography revealed non-specific ST segment and T wave changes in the precordial leads, but no ST segment elevation. Cardiac biomarkers (cardiac troponin I and creatine kinase MB fraction) were elevated (cardiac troponin I measured 0.12 µg/mL; institutional upper limit of normal ≥0.1 µg/mL). After 2 days in the critical care unit, the patient was transferred to the cath lab. A coronary angiography via radial approach revealed a calcified type-B2 focal lesion in the mid-segment of a large-sized left anterior descending coronary artery (LAD), causing 95% luminal obstruction. Coronary angioplasty was performed under cover of bivalirudin (intravenous bolus followed by infusion). The patient received 600 mg of oral clopidogrel as a loading dose immediately following the procedure, according to our institutional protocol. Predilatation with a cutting balloon was initially performed, followed by the successful implantation of a 3.5 mm x 12 mm everolimus-eluting stent (Xience V, Abbott Vascular), deployed at 12 atm. In order to optimize the result in a calcified lesion, the operator performed post-dilatation with a 3.5 mm x 8 mm balloon inflated at 20 atm with a final TIMI grade 3 flow and 20% residual stenosis. The patient had an uncomplicated in-hospital course and was prescribed aspirin 100 mg daily indefinitely, and clopidogrel 75 mg daily, for 6 months after discharge (according to our institutional protocol following drug-eluting stent implantation). In a follow-up visit in August 2009, the patient was symptom-free, and echocardiography demonstrated a left ventricular ejection fraction of 70%, a left ventricular end-diastolic diameter of 55 mm, and no regional wall motion abnormalities.
Case Report. A 62-year-old male patient presented to our emergency department in June 2009 with persistent ischemic-type chest pain for 3 H. He was an insulin-dependent diabetic, hypertensive, dyslipidemic, with chronic renal impairment. Electrocardiography revealed non-specific ST segment and T wave changes in the precordial leads, but no ST segment elevation. Cardiac biomarkers (cardiac troponin I and creatine kinase MB fraction) were elevated (cardiac troponin I measured 0.12 µg/mL; institutional upper limit of normal ≥0.1 µg/mL). After 2 days in the critical care unit, the patient was transferred to the cath lab. A coronary angiography via radial approach revealed a calcified type-B2 focal lesion in the mid-segment of a large-sized left anterior descending coronary artery (LAD), causing 95% luminal obstruction. Coronary angioplasty was performed under cover of bivalirudin (intravenous bolus followed by infusion). The patient received 600 mg of oral clopidogrel as a loading dose immediately following the procedure, according to our institutional protocol. Predilatation with a cutting balloon was initially performed, followed by the successful implantation of a 3.5 mm x 12 mm everolimus-eluting stent (Xience V, Abbott Vascular), deployed at 12 atm. In order to optimize the result in a calcified lesion, the operator performed post-dilatation with a 3.5 mm x 8 mm balloon inflated at 20 atm with a final TIMI grade 3 flow and 20% residual stenosis. The patient had an uncomplicated in-hospital course and was prescribed aspirin 100 mg daily indefinitely, and clopidogrel 75 mg daily, for 6 months after discharge (according to our institutional protocol following drug-eluting stent implantation). In a follow-up visit in August 2009, the patient was symptom-free, and echocardiography demonstrated a left ventricular ejection fraction of 70%, a left ventricular end-diastolic diameter of 55 mm, and no regional wall motion abnormalities.
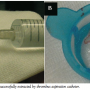
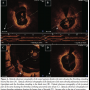 In January 2010, the patient discontinued clopidogrel. After 2 weeks, he experienced recurrent angina (Canadian Cardiovascular Society score II) and was admitted once again to our critical care unit. Nevertheless, electrocardiography was unremarkable, and cardiac biomarkers were within the normal range. In the context of multiple risk factors, the previously implanted stent, and more importantly, the recent discontinuation of clopidogrel, he was triaged for invasive study. Interestingly, coronary angiography revealed normal opacification of the index artery with no in-stent restenosis, and notably, no evidence of thrombosis (Figure 1). Obviously driven by a high index of suspicion, the operator decided to explore the artery with optical coherence tomography (OCT). Anticoagulation was achieved by enoxaparin 1 mg/kg intravenously (90 mg). Surprisingly, OCT (LightLab Imaging Inc.) discovered a ribbon-like thrombus starting in the proximal part of the stent and extending beyond its distal part. OCT was also able to clearly demonstrate a substantial proportion of uncovered and malapposed stent struts in the proximal part of the stent and a good deal of neointimal hyperplasia in its distal part (Figures 2A-2C). After a guidewire passage, thrombectomy was effectively performed using a thrombus-aspiration catheter, and a red thrombus was eventually extracted (Figure 3). Following thrombectomy, OCT confirmed adequate removal of the thrombus (Figure 2D). No balloon angioplasty was performed for stent malapposition because only a few malapposed struts were observed. Cardiac biomarkers were persistently negative at 6- and 12-hour intervals. The patient was kept on dual antiplatelet therapy in the form of aspirin 100 mg/d, and clopidogrel 75 mg/d for 12 months.
In January 2010, the patient discontinued clopidogrel. After 2 weeks, he experienced recurrent angina (Canadian Cardiovascular Society score II) and was admitted once again to our critical care unit. Nevertheless, electrocardiography was unremarkable, and cardiac biomarkers were within the normal range. In the context of multiple risk factors, the previously implanted stent, and more importantly, the recent discontinuation of clopidogrel, he was triaged for invasive study. Interestingly, coronary angiography revealed normal opacification of the index artery with no in-stent restenosis, and notably, no evidence of thrombosis (Figure 1). Obviously driven by a high index of suspicion, the operator decided to explore the artery with optical coherence tomography (OCT). Anticoagulation was achieved by enoxaparin 1 mg/kg intravenously (90 mg). Surprisingly, OCT (LightLab Imaging Inc.) discovered a ribbon-like thrombus starting in the proximal part of the stent and extending beyond its distal part. OCT was also able to clearly demonstrate a substantial proportion of uncovered and malapposed stent struts in the proximal part of the stent and a good deal of neointimal hyperplasia in its distal part (Figures 2A-2C). After a guidewire passage, thrombectomy was effectively performed using a thrombus-aspiration catheter, and a red thrombus was eventually extracted (Figure 3). Following thrombectomy, OCT confirmed adequate removal of the thrombus (Figure 2D). No balloon angioplasty was performed for stent malapposition because only a few malapposed struts were observed. Cardiac biomarkers were persistently negative at 6- and 12-hour intervals. The patient was kept on dual antiplatelet therapy in the form of aspirin 100 mg/d, and clopidogrel 75 mg/d for 12 months.
 In May 2010, the patient started to develop exertional dyspnea (NYHA class I) but no chest pain. Echocardiography at this point demonstrated a left ventricular ejection fraction of 65%, a left ventricular end-diastolic diameter of 56 mm, and again no regional wall motion abnormalities. In view of the background history of stent thrombosis, stress radionuclide scintigraphy was performed one month later. Favorably, the patient achieved his target heart rate for age with no chest pain, or abnormal electrocardiographic changes. Radionuclide scintigraphy demonstrated a mild reversible perfusion defect in the territory of the left circumflex coronary artery. Fortunately, there was no ischemia in the LAD territory. Finally, scheduled follow-up coronary angiography was performed in September 2010 and assured a patent stent with adequate flow. More importantly, OCT performed along with coronary angiography confirmed the absence of further thrombi.
In May 2010, the patient started to develop exertional dyspnea (NYHA class I) but no chest pain. Echocardiography at this point demonstrated a left ventricular ejection fraction of 65%, a left ventricular end-diastolic diameter of 56 mm, and again no regional wall motion abnormalities. In view of the background history of stent thrombosis, stress radionuclide scintigraphy was performed one month later. Favorably, the patient achieved his target heart rate for age with no chest pain, or abnormal electrocardiographic changes. Radionuclide scintigraphy demonstrated a mild reversible perfusion defect in the territory of the left circumflex coronary artery. Fortunately, there was no ischemia in the LAD territory. Finally, scheduled follow-up coronary angiography was performed in September 2010 and assured a patent stent with adequate flow. More importantly, OCT performed along with coronary angiography confirmed the absence of further thrombi.
Discussion
In our case, the patient developed an acute coronary syndrome 2 weeks following the discontinuation of clopidogrel. He received dual antiplatelet therapy for no more than 6 months following the implantation of an everolimus-eluting stent. The world leading authorities of guidelines have already recommended extending the duration of dual antiplatelet therapy following implantation of first-generation drug-eluting stent (DES) up to 1 year.1 The currently marketed DES bear the same inherent problem of a stent platform surrounded by a polymer that releases a highly aggressive drug and therefore, would carry the same risk of inadequate arterial healing and potentially subsequent stent thrombosis. Eventually, the growing evidence demonstrating late-occurring unpredictable major events following implantation of DES might further push the guidelines' committees to recommend continuing the drug-dependent state indefinitely. Under these circumstances, surgery that needs discontinuation of this therapy would pose an imminent risk of a hard endpoint. Ongoing trials are currently underway in order to clearly identify the optimal duration of dual antiplatelet therapy following DES.2,3
As displayed by OCT, a great proportion of stent struts were still uncovered 6 months following DES implantation in our patient. This probably would have created a favorable milieu for stent thrombosis to occur once the antiplatelet therapy was reduced by withdrawal of clopidogrel. The specific diagnostic value of OCT lies in its ability to provide unique information on the stent interaction with the vessel wall at the level of individual struts. A florid example of this appears in evaluating neointimal coverage following coronary stenting. In this concern, OCT can reliably visualize early and very thin layers of neointimal coverage over stent struts, and can quantify them with high reproducibility.4 This renders OCT an ideal technique to compare subtle, yet relevant differences in neointimal coverage between various stent designs.5-7 Furthermore, in spite of post-dilatation at a high pressure, there was a high proportion of malapposed struts in the proximal part of the stent. This might raise the issue of late-acquired strut malapposition. Plaque reduction behind the stent (due to thrombus resolution or plaque regression), positive remodeling of the vessel wall, or chronic stent recoil, are potential mechanisms that have been put forward to account for this mysterious phenomenon.8 Additionally, our case would make a strong argument for the value of coronary OCT as an indispensable tool in clinical decision-making whenever there is discrepancy between symptoms and coronary angiographic findings after stent implantation. Despite the fact that coronary angiography was uneventful when our patient presented with chest pain for the second time, OCT effectively provided clear evidence for an ongoing thrombotic process.
Clinical Implications
Lack of neointimal coverage of stent struts (and possibly stent strut malapposition) might provide a potential substrate for late-occurring stent thrombosis following implantation of DES. In this regard, the decision on whether to extend dual antiplatelet therapy will have to await the results of ongoing randomized controlled trials comparing varying lengths of therapy following DES implantation.2,3
References
- King SB 3rd, Smith SC Jr, Hirshfeld JW Jr, et al. 2007 focused update of the ACC/AHA/SCAI 2005 guideline update for percutaneous coronary intervention: a report of the American College of Cardiology/American Heart Association Task Force on Practice guidelines. J Am Coll Cardiol. 2008;51(2):172-209.
- Mauri L, Kereiakes DJ, Normand SL, et al. Rationale and design of the dual antiplatelet therapy study, a prospective, multicenter, randomized, double-blind trial to assess the effectiveness and safety of 12 versus 30 months of dual antiplatelet therapy in subjects undergoing percutaneous coronary intervention with either drug-eluting stent or bare metal stent placement for the treatment of coronary artery lesions. Am Heart J. 2010;160(6):1035-1041, 1041.e1.
- Byrne RA, Schulz S, Mehilli J, et al; for the Intracoronary Stenting and Antithrombotic Regimen: Safety And EFficacy of Six Months Dual Antiplatelet Therapy After Drug-Eluting Stenting (ISAR-SAFE) Investigators. Rationale and design of a randomized, double-blind, placebo-controlled trial of 6 versus 12 months clopidogrel therapy after implantation of a drug-eluting stent: The Intracoronary Stenting and Antithrombotic Regimen: Safety And EFficacy of 6 Months Dual Antiplatelet Therapy After Drug-Eluting Stenting (ISAR-SAFE) study. Am Heart J. 2009;157(4):620-624.e2.
- Tanimoto S, Rodriguez-Granillo G, Barlis P, et al. A novel approach for quantitative analysis of intracoronary optical coherence tomography: high inter-observer agreement with computer-assisted contour detection. Catheter Cardiovasc Interv. 2008;72(2):228-235.
- Barlis P, Regar E, Serruys PW, et al. An optical coherence tomography study of a biodegradable vs. durable polymer-coated limus-eluting stent: a LEADERS trial sub-study. Eur Heart J. 2010;31(2):165-176.
- Guagliumi G, Costa MA, Sirbu V, et al. Strut coverage and late malapposition with paclitaxel-eluting stents compared with bare metal stents in acute myocardial infarction: optical coherence tomography substudy of the Harmonizing Outcomes with Revascularization and Stents in Acute Myocardial Infarction (HORIZONS-AMI) Trial. Circulation. 2011;123(3):274-281.
- Gutiérrez-Chico JL, van Geuns RJ, Regar E, et al. Tissue coverage of a hydrophilic polymer-coated zotarolimus-eluting stent vs. a fluoropolymer-coated everolimus-eluting stent at 13-month follow-up: an optical coherence tomography substudy from the RESOLUTE All Comers trial. Eur Heart J. 2011;32(19):2454-2463.
- Hassan AK, Bergheanu SC, Stijnen T, et al. Late stent malapposition risk is higher after drug-eluting stent compared with bare-metal stent implantation and associates with late stent thrombosis. Eur Heart J. 2010;31(10):1172-1180.
_________________________________________________________
From the Department of Cardiology, Satakunta Central Hospital, Sairaalantie 3, FIN-28100, Pori, Finland.
Disclosure: The authors have completed and returned the ICMJE Form for Disclosure of Potential Conflicts of Interest. The authors report no conflicts of interest regarding the content herein.
Manuscript submitted March 8, 2012, provisional acceptance given April 23, 2012, final version accepted May 14, 2012.
Address for correspondence: Dr. Pasi P Karjalainen, Department of Cardiology, Satakunta Central Hospital, Sairaalantie 3, FIN-28100, Pori, Finland. E-mail: pasi.karjalainen@satshp.fi











